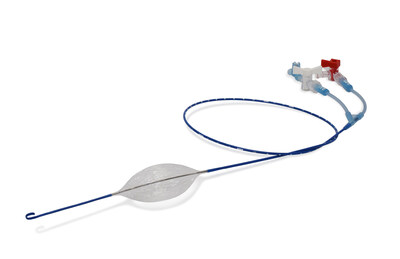
Prytime Medical Devices Receives World's First Extended Duration FDA 510(k) Clearance for pREBOA-PRO™ Catheter
PR Newswire
BOERNE, Texas, Oct. 8, 2025
BOERNE, Texas, Oct. 8, 2025 /PRNewswire/ -- Building once again on their first mover position as a leader in endovascular hemorrhage control and resuscitation, Prytime Medical Devices, Inc. (Prytime) this week announced a new FDA 510(k) clearance for their flagship pREBOA-PRO™ Catheter.
The new clearance includes a dramatically expanded use profile which provides safe occlusion times up to 2 Hours in Zone 1, a four time increase from all other occlusion catheters. In addition, the new clearance contains an industry defining formal definition for partial occlusion.
REBOA has traditionally been used reactively and often as a last resort, due to extremely limited 30-minute safe occlusion times. This week's announcement highlights the evolving new capabilities now available to surgeons with the world's first and only purpose-built partial balloon occlusion technology.
The patented pREBOA-PRO™ Catheter is designed with flow channels which enable precise, smooth manual control of patient blood flow. PRO's ability to precisely control arterial flow enables surgeons to immediately stabilize blood pressure and reduce blood loss while simultaneously diminishing the risk of distal ischemia and detrimental reperfusion sequelae associated with old REBOA.
"This new capability was designed with the surgeon in mind and helps eliminate a major barrier to broader utility. With this new pREBOA-PRO™ catheter, our surgical teams can safely intervene earlier and gain a new level of control, especially during the initial chaos seen in challenging trauma cases. Gaining control early reduces the collective temperature in the room and provides critical extended time to get to definitive intervention," said Dr. Chance Spalding, a Trauma and Critical Care Surgeon at Mount Carmel East Hospital in Columbus, Ohio and Prytime's Chief Medical Officer.
David Spencer, CEO of Prytime Medical adds, "This advanced partial occlusion technology was designed to give surgeons the extended time and hemorrhage control they've been asking for. Individually and collectively, surgeons pointed out deficiencies with traditional REBOA catheters. At Prytime we listened, and we fixed them. Partial occlusion is not just letting fluid out of a balloon. By utilizing this new purpose-built partial occlusion design, physicians get a strongly enhanced risk profile while extending the window for definitive surgical repair through simple, safe flow control," Spencer said. "pREBOA-PRO™ is the world's first and only catheter to give physicians the control they've been asking for."
For more information, see www.prytimemedical.com or email sales@prytimemedical.com.
ABOUT PRYTIME MEDICAL, INC.
Prytime Medical Inc. is a leading innovator and global provider of lifesaving endovascular occlusion products. Our mission is to provide cutting-edge solutions and training designed to give physicians clinical optionality and better save lives by safely controlling blood flow while preventing ischemic complications. Use our products to "Buy Time, Gain Control."
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/prytime-medical-devices-receives-worlds-first-extended-duration-fda-510k-clearance-for-preboa-pro-catheter-302578630.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/prytime-medical-devices-receives-worlds-first-extended-duration-fda-510k-clearance-for-preboa-pro-catheter-302578630.html
SOURCE Prytime Medical